Chemical Datasheet
SULFUR, MOLTEN |

|
Chemical Identifiers
The
Chemical Identifier fields
include common identification numbers, the
NFPA diamond
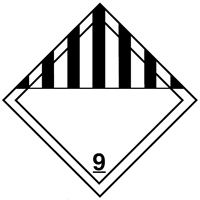
U.S. Department of Transportation hazard labels, and a general
description of the chemical. The information in CAMEO Chemicals comes
from a variety of
data sources.
| CAS Number | UN/NA Number | DOT Hazard Label | USCG CHRIS Code |
|---|---|---|---|
|
|
||
| NIOSH Pocket Guide | International Chem Safety Card | ||
| none | |||
NFPA 704
| Diamond | Hazard | Value | Description | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
2 | Can cause temporary incapacitation or residual injury. | |||||||||
|
|
1 | Must be preheated before ignition can occur. | ||||||||||
|
|
0 | Normally stable, even under fire conditions. | ||||||||||
|
|
(NFPA, 2010)
General Description
A pale yellow crystalline solid with a faint odor of rotten eggs. Insoluble in water. A fire and explosion risk above 450°F. Transported as a yellow to red liquid. Handled at elevated temperature (typically 290°F) to prevent solidification and makes transfers easier. Hot enough that plastic or rubber may melt or lose strength. Causes thermal burns to skin on contact. Cools rapidly and solidifies if released. Equipment designed to protect against ordinary chemical exposure is ineffective against the thermal hazard. Exercise caution walking on the surface of a spill to avoid breakthrough into pockets of molten sulfur below the crust. Do not attempt to remove sulfur impregnated clothing because of the danger of tearing flesh if a burn has resulted. May be irritatin to skin, eyes and mucous membranes. Used in sulfuric acid production, petroleum refining, and pulp and paper manufacturing.
Hazards
The
Hazard fields
include
special hazard alerts
air and water
reactions, fire hazards, health hazards, a reactivity profile, and
details about
reactive groups assignments
and
potentially incompatible absorbents.
The information in CAMEO Chemicals comes from a variety of
data sources.
Reactivity Alerts
- Highly Flammable
- Strong Reducing Agent
Air & Water Reactions
Flammable. Insoluble in water.
Fire Hazard
Special Hazards of Combustion Products: Produces toxic sulfur dioxide gas.
Behavior in Fire: Burns with a pale blue flame that may be difficult to see in daylight. (USCG, 1999)
Behavior in Fire: Burns with a pale blue flame that may be difficult to see in daylight. (USCG, 1999)
Health Hazard
Can cause eye irritation; may rarely irritate skin. If recovered sulfur, refer to hydrogen sulfide.* (USCG, 1999)
Reactivity Profile
SULFUR reacts violently with strong oxidizing agents causing fire and explosion hazards [Handling Chemicals Safely 1980 p. 871]. Reacts with iron to give pyrophoric compounds. Attacks copper, silver and mercury. Reacts with bromine trifluoride, even at 10°C [Mellor 2:113. 1946-47]. Ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. Reacts to incandescence with heated with thorium [Mellor 7:208 1946-47]. Can react with ammonia to form explosive sulfur nitride. Reacts with calcium phosphide incandescently at about 300°C. Reacts violently with phosphorus trioxide [Chem. Eng. News 27:2144 1949]. Mixtures with ammonium nitrate or with metal powders can be exploded by shock [Kirk and Othmer 8:644]. Combinations of finely divided sulfur with finely divided bromates, chlorates, or iodates of barium, calcium, magnesium, potassium, sodium, or zinc can explode with heat, friction, percussion, and sometimes light [Mellor 2 Supp.1:763. 1956]. A mixture with barium carbide heated to 150°C becomes incandescent. Reacts incandescently with calcium carbide or strontium carbide at 500°C. Attacks heated lithium, or heated selenium carbide with incandescence [Mellor 5:862 1946-47]. Reacts explosively if warmed with powdered zinc [Mellor 4:476. 1946-47]. Reacts vigorously with tin [Mellor 7:328. 1946-47]. A mixture with potassium nitrate and arsenic trisulfide is a known pyrotechnic formulation [Ellern 1968 p. 135]. Mixtures with any perchlorate can explode on impact [ACS 146:211-212]. A mixture of damp sulfur and calcium hypochlorite produces a brilliant crimson flash with scatter of molten sulfur [Chem. Eng. News 46(28):9 1968]. Takes fire spontaneously in chlorine dioxide and may produce an explosion [Mellor 2:289 (1946-47)]. Ignites if heated with chromic anhydride ignite and can explode, [Mellor 10:102 (1946-47)]. Even small percentages of hydrocarbons in contact with molten sulfur generate hydrogen sulfide and carbon disulfide, which may accumulate in explosive concentrations. Sulfur reacts with Group I metal nitrides to form flammable mixtures, evolving flammable and toxic NH3 and H2S gases if water is present (Mellor, 1940, Vol. 8, 99).
Belongs to the Following Reactive Group(s)
Potentially Incompatible Absorbents
No information available.
Response Recommendations
The
Response Recommendation fields
include isolation and evacuation distances, as well as recommendations for
firefighting, non-fire response, protective clothing, and first aid. The
information in CAMEO Chemicals comes from a variety of
data sources.
Isolation and Evacuation
Excerpt from ERG Guide 133 [Flammable Solids]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area for at least 25 meters (75 feet) in all directions.
LARGE SPILL: Consider initial downwind evacuation for at least 100 meters (330 feet).
FIRE: If tank, rail car or tank truck is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2020)
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area for at least 25 meters (75 feet) in all directions.
LARGE SPILL: Consider initial downwind evacuation for at least 100 meters (330 feet).
FIRE: If tank, rail car or tank truck is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2020)
Firefighting
Excerpt from ERG Guide 133 [Flammable Solids]:
SMALL FIRE: Dry chemical, CO2, sand, earth, water spray or regular foam.
LARGE FIRE: Water spray, fog or regular foam. If it can be done safely, move undamaged containers away from the area around the fire. Fire Involving Metal Pigments or Pastes (e.g. "Aluminum Paste") Aluminum Paste fires should be treated as a combustible metal fire. Use DRY sand, graphite powder, dry sodium chloride-based extinguishers or class D extinguishers. Also, see ERG Guide 170.
FIRE INVOLVING TANKS OR CAR/TRAILER LOADS: Cool containers with flooding quantities of water until well after fire is out. For massive fire, use unmanned master stream devices or monitor nozzles; if this is impossible, withdraw from area and let fire burn. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks engulfed in fire. (ERG, 2020)
SMALL FIRE: Dry chemical, CO2, sand, earth, water spray or regular foam.
LARGE FIRE: Water spray, fog or regular foam. If it can be done safely, move undamaged containers away from the area around the fire. Fire Involving Metal Pigments or Pastes (e.g. "Aluminum Paste") Aluminum Paste fires should be treated as a combustible metal fire. Use DRY sand, graphite powder, dry sodium chloride-based extinguishers or class D extinguishers. Also, see ERG Guide 170.
FIRE INVOLVING TANKS OR CAR/TRAILER LOADS: Cool containers with flooding quantities of water until well after fire is out. For massive fire, use unmanned master stream devices or monitor nozzles; if this is impossible, withdraw from area and let fire burn. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks engulfed in fire. (ERG, 2020)
Non-Fire Response
Excerpt from ERG Guide 133 [Flammable Solids]:
ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area. Do not touch or walk through spilled material.
SMALL DRY SPILL: With clean shovel, place material into clean, dry container and cover loosely; move containers from spill area.
LARGE SPILL: Wet down with water and dike for later disposal. Prevent entry into waterways, sewers, basements or confined areas. (ERG, 2020)
ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area. Do not touch or walk through spilled material.
SMALL DRY SPILL: With clean shovel, place material into clean, dry container and cover loosely; move containers from spill area.
LARGE SPILL: Wet down with water and dike for later disposal. Prevent entry into waterways, sewers, basements or confined areas. (ERG, 2020)
Protective Clothing
Safety goggles with side shields; approved respirator; heat-resistant gloves; leather heat-resistant clothing. If recovered sulfur, refer to hydrogen sulfide.* (USCG, 1999)
DuPont Tychem® Suit Fabrics
No information available.
First Aid
EYES: wash eyes carefully for at least 15 min.
SKIN: Treat molten sulfur burns with petroleum jelly or mineral oil. If recovered sulfur, treat as for hydrogen sulfide.* (USCG, 1999)
SKIN: Treat molten sulfur burns with petroleum jelly or mineral oil. If recovered sulfur, treat as for hydrogen sulfide.* (USCG, 1999)
Physical Properties
The
Physical Property fields
include properties such as vapor pressure and
boiling point, as well as explosive limits and
toxic exposure thresholds
The information in CAMEO Chemicals comes from a variety of
data sources.
Note: For Vapor Density and Specific Gravity, comparing the value to 1.0 can tell you if the chemical will likely sink/rise in air or sink/float in fresh water (respectively). Short phrases have been added to those values below as an aid. However, make sure to also consider the circumstances of a release. The Vapor Density comparisons are only valid when the gas escaping is at the same temperature as the surrounding air itself. If the chemical is escaping from a container where it was pressurized or refrigerated, it may first escape and behave as a heavy gas and sink in the air (even if it has a Vapor Density value less than 1). Also, the Specific Gravity comparisons are for fresh water (density 1.0 g/mL). If your spill is in salt water (density about 1.027 g/mL), you need to adjust the point of comparison. There are some chemicals that will sink in fresh water and float in salt water.
Note: For Vapor Density and Specific Gravity, comparing the value to 1.0 can tell you if the chemical will likely sink/rise in air or sink/float in fresh water (respectively). Short phrases have been added to those values below as an aid. However, make sure to also consider the circumstances of a release. The Vapor Density comparisons are only valid when the gas escaping is at the same temperature as the surrounding air itself. If the chemical is escaping from a container where it was pressurized or refrigerated, it may first escape and behave as a heavy gas and sink in the air (even if it has a Vapor Density value less than 1). Also, the Specific Gravity comparisons are for fresh water (density 1.0 g/mL). If your spill is in salt water (density about 1.027 g/mL), you need to adjust the point of comparison. There are some chemicals that will sink in fresh water and float in salt water.
| Chemical Formula: |
|
Flash Point:
405°F
(USCG, 1999)
Lower Explosive Limit (LEL): data unavailable
Upper Explosive Limit (UEL): data unavailable
Autoignition Temperature:
450°F
(USCG, 1999)
Melting Point:
251°F
(USCG, 1999)
Vapor Pressure: data unavailable
Vapor Density (Relative to Air): data unavailable
Specific Gravity:
1.8
at 248°F
(USCG, 1999)
- Denser than water; will sink
Boiling Point:
832.3°F
at 760 mmHg
(USCG, 1999)
Molecular Weight:
256.51
(USCG, 1999)
Water Solubility: data unavailable
Ionization Energy/Potential: data unavailable
IDLH: data unavailable
AEGLs (Acute Exposure Guideline Levels)
No AEGL information available.ERPGs (Emergency Response Planning Guidelines)
No ERPG information available.PACs (Protective Action Criteria)
No PAC information available.Regulatory Information
The
Regulatory Information fields
include information from
the U.S. Environmental Protection Agency's Title III Consolidated List of
Lists,
the U.S. Cybersecurity and Infrastructure Security Agency's Chemical Facility
Anti-Terrorism Standards,
and the U.S. Occupational Safety and Health Administration's
Process Safety Management of Highly Hazardous Chemicals Standard List
(see more about these
data sources).
EPA Consolidated List of Lists
No regulatory information available.CISA Chemical Facility Anti-Terrorism Standards (CFATS)
No regulatory information available.OSHA Process Safety Management (PSM) Standard List
No regulatory information available.Alternate Chemical Names
This section provides a listing of alternate names for this chemical,
including trade names and synonyms.
- BRIMSTONE
- SULFUR
- SULFUR, DRY
- SULFUR, MOLTEN
- SULFUR, [MOLTEN]
- SULFUR, [SOLID]
- SULPHUR
- SULPHUR, MOLTEN